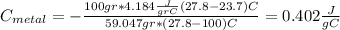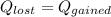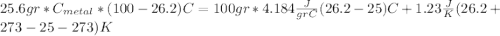For the following scenarios what is the metal? a piece of metal weighing 59.047 g was heated to 100.0 degree c and then put it into 100.0 ml of water (initially at 23.7 degree c). the metal and water were allowed to come to an equilibrium temperature, determined to be 27.8 degree c. assuming no heat lost to the environment, calculate the specific heat of the metal. a 25.6 g piece of metal was taken from a beaker of boiling water at 100.0 degree c and placed directly into a calorimeter holding 100.0 ml of water at 25.0 degree c. the calorimeter heat capacity is 1.23 j/k. given that the final temperature at thermal equilibrium is 26.2 degree c, determine the specific heat capacity of the metal.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 23.06.2019 14:30
The atomic number of an element is based on the number of electrons around its core mass of its nucleus number of protons in its nucleus number of neutrons in its nucleus
Answers: 2

Chemistry, 23.06.2019 18:30
Sediment that settles on the bottom of the sea may form layers of rock which description tells how this sediment is deposited. a.)randomely b.)vertically c.)diagonally d.)horizontally
Answers: 2

Chemistry, 23.06.2019 18:50
Which of the following elements is most likely to have an oxidation state of +2? a. oxygen (0) b. sodium (na) c. chlorine (ci) d. strontium (si)
Answers: 1
You know the right answer?
For the following scenarios what is the metal? a piece of metal weighing 59.047 g was heated to 100...
Questions


Geography, 04.08.2019 23:30


History, 04.08.2019 23:30

History, 04.08.2019 23:30




Mathematics, 04.08.2019 23:30

English, 04.08.2019 23:30

Mathematics, 04.08.2019 23:30

Biology, 04.08.2019 23:30

Geography, 04.08.2019 23:30

Geography, 04.08.2019 23:30



Chemistry, 04.08.2019 23:30

Mathematics, 04.08.2019 23:30

Mathematics, 04.08.2019 23:30

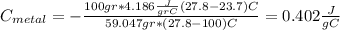
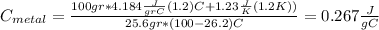
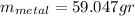 the mass of the metal
the mass of the metal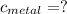 Is the value that we need to find
Is the value that we need to find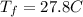 represent the final temperature of equilibrium for the metal and the water
represent the final temperature of equilibrium for the metal and the water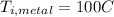 represent the initial temperature for the metal
represent the initial temperature for the metal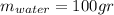 since the density is 1g/ml
since the density is 1g/ml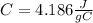 the specific heat for the liquid water
the specific heat for the liquid water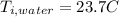 the initial temperature for the water
the initial temperature for the water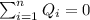 if we have balance then we have this:
if we have balance then we have this: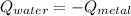
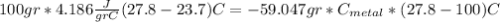
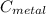 we got:
we got: