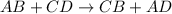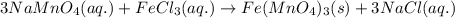Sodium permanganate and iron(iii) chloride are both dissolved in a beaker of water. in a double displacement reaction, two new products are formed. a. write the complete balanced chemical equation (using chemical formulas, not words) for this reaction. include the physical states of the reactants and the products

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

You know the right answer?
Sodium permanganate and iron(iii) chloride are both dissolved in a beaker of water. in a double disp...
Questions

English, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01


Mathematics, 20.09.2020 17:01


Chemistry, 20.09.2020 17:01


English, 20.09.2020 17:01

English, 20.09.2020 17:01



Mathematics, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01

Biology, 20.09.2020 17:01


History, 20.09.2020 17:01

Geography, 20.09.2020 17:01