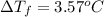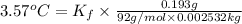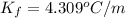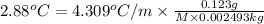Chemistry, 24.01.2020 18:31 Braxtonw875
1) if 0.193 grams of toluene is dissolved in 2.532 grams of p-xylene, what is the molality of toluene in the solution? 2) if a freezing point depression of 3.57°celcius is measured for the solution described in question 1, calculate  for p-xylene.3) suppose you dissolved 0.123 gram of pentane in 2.493 grams of p-xylene and measured a freezing point depression of 2.88°celcius for the solution. calculate the molar mass of pentane using this data and the value for
for p-xylene.3) suppose you dissolved 0.123 gram of pentane in 2.493 grams of p-xylene and measured a freezing point depression of 2.88°celcius for the solution. calculate the molar mass of pentane using this data and the value for  that you calculated in question 2.
that you calculated in question 2.

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 10:20
An engineer wishes to design a container that will hold 12.0 mol of ethane at a pressure no greater than 5.00x10*2 kpa and a temperature of 52.0 degrees celsius. what is the minimum volume the container can have?
Answers: 1

Chemistry, 23.06.2019 11:00
Which example is a mechanical wave? a.microwave b.radio wave c.water wave d.ultraviolet light
Answers: 1

Chemistry, 23.06.2019 11:30
Bridget is in science class. her teacher gives her two unknown substances and asks her to determine their relative ph. she places a piece of red litmus paper into both substances. the litmus paper turns purple when she places it into substance i. the litmus paper turns blue when she places it into substance ii. a. substance i is a neutral substance and substance ii is an acid. b. substance i is a neutral substance and substance ii is a base. c. substance i is an acid and substance ii is a base. d. substance i is a base and substance ii is a neutral substance.
Answers: 1
You know the right answer?
1) if 0.193 grams of toluene is dissolved in 2.532 grams of p-xylene, what is the molality of toluen...
Questions


History, 14.10.2020 14:01




Engineering, 14.10.2020 14:01


Mathematics, 14.10.2020 14:01



Computers and Technology, 14.10.2020 14:01

Mathematics, 14.10.2020 14:01

Mathematics, 14.10.2020 14:01


Mathematics, 14.10.2020 14:01

Law, 14.10.2020 14:01

Mathematics, 14.10.2020 14:01


Mathematics, 14.10.2020 14:01

Chemistry, 14.10.2020 14:01

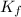 for xylene is 4.309°C/m.
for xylene is 4.309°C/m.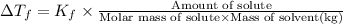
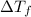 =depression in freezing point
=depression in freezing point