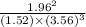Chemistry, 24.01.2020 10:31 cheervolley
A1.000 l vessel is filled with 1.000 mole of n2,2.000 moles of h2, and 3.000 moles of nh3. when the reaction n2(g) + 3 h2(g)⇀↽2 nh3(g) comes to equilibrium, it is observed that the concentration of nh3is 2.12 moles/l. what is the numerical value of the equilibrium constant kc?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1
You know the right answer?
A1.000 l vessel is filled with 1.000 mole of n2,2.000 moles of h2, and 3.000 moles of nh3. when the...
Questions

Physics, 11.05.2021 15:50




Biology, 11.05.2021 15:50


Spanish, 11.05.2021 15:50


Mathematics, 11.05.2021 15:50


Mathematics, 11.05.2021 15:50

Mathematics, 11.05.2021 15:50


History, 11.05.2021 15:50

Mathematics, 11.05.2021 15:50


English, 11.05.2021 15:50


Mathematics, 11.05.2021 15:50

Mathematics, 11.05.2021 15:50

![[NH^3]= \frac{3mol}{1l}](/tpl/images/0468/7747/69ad7.png) = 3M
= 3M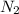 ] = 1M
] = 1M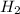 ] = 2 M
] = 2 M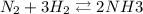
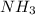 ] = 1.96 M
] = 1.96 M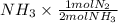 ) = 0.52 mol created (in addition to 1 mol already in vessel)
) = 0.52 mol created (in addition to 1 mol already in vessel)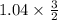 1.56 moles created
1.56 moles created 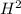 ] = 1.56 + 2 = 3.56 M
] = 1.56 + 2 = 3.56 M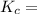
![\frac{[NH^3]^2}{N^2[H^2]^3}](/tpl/images/0468/7747/7f945.png)