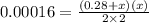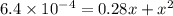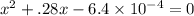Chemistry, 24.01.2020 10:31 matthewquattlebum
When solid nh4hs and 0.28 mol nh3(g) were placed in a 2 l vessel at 24◦c, the equilibriumnh4hs(s)⇀↽nh3(g) + h2s(g)for which kc= 0.00016, was reached. what is the equilibrium concentration of nh3? answer in units of mol/l

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2


Chemistry, 23.06.2019 12:20
Amatch has about 21 milligrams of red phosphorus coating the tip. how many atoms of phosphorus is this?
Answers: 1
You know the right answer?
When solid nh4hs and 0.28 mol nh3(g) were placed in a 2 l vessel at 24◦c, the equilibriumnh4hs(s)⇀↽n...
Questions

Geography, 10.03.2021 19:50


Mathematics, 10.03.2021 19:50



Mathematics, 10.03.2021 19:50




Mathematics, 10.03.2021 19:50

Social Studies, 10.03.2021 19:50


Mathematics, 10.03.2021 19:50



Mathematics, 10.03.2021 19:50


Chemistry, 10.03.2021 19:50

Mathematics, 10.03.2021 19:50


![Kc=[NH_{3}][H_{2}S]](/tpl/images/0468/7808/2a772.png)

![[NH_{3}]=\frac{0.28 +x}{2}](/tpl/images/0468/7808/aa8ed.png)
![[H_{2}S]=\frac{x}{2}](/tpl/images/0468/7808/ba461.png)