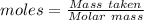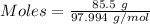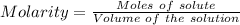Chemistry, 24.01.2020 02:31 andrewlawton8125
Aconcentrated phosphoric acid solution is 85.5% h_3po_4 by mass and has a density of 1.69 g/ml at 25 degree c. what is the molarity of h_3po_4? a. 14.7 m b 0.166 c. 5.16 m d. 19.4 m e. 0.0516 m

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
Aconcentrated phosphoric acid solution is 85.5% h_3po_4 by mass and has a density of 1.69 g/ml at 25...
Questions



Mathematics, 02.07.2019 08:30

Mathematics, 02.07.2019 08:30

English, 02.07.2019 08:30





Mathematics, 02.07.2019 08:30

Social Studies, 02.07.2019 08:30



History, 02.07.2019 08:30

Mathematics, 02.07.2019 08:30

Physics, 02.07.2019 08:30

Physics, 02.07.2019 08:30


Mathematics, 02.07.2019 08:30

Physics, 02.07.2019 08:30

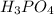 is 85.5 % w/w in solution.
is 85.5 % w/w in solution.