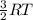Chemistry, 24.01.2020 02:31 ineedtopeebeforethec
Consider two gases, a and b, that are in a container at room temperature. what effect will the following change have on the rate of the reaction between these gases?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
Consider two gases, a and b, that are in a container at room temperature. what effect will the follo...
Questions


Mathematics, 16.05.2021 22:50


Mathematics, 16.05.2021 22:50

World Languages, 16.05.2021 22:50


Health, 16.05.2021 22:50

Mathematics, 16.05.2021 22:50


English, 16.05.2021 22:50


English, 16.05.2021 22:50


Mathematics, 16.05.2021 22:50



World Languages, 16.05.2021 22:50


English, 16.05.2021 22:50