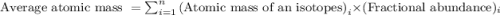Chemistry, 24.01.2020 01:31 ashkids7178
The element copper has two stable isotopes, copper-63 with a mass of 62.93 amu and copper-65 with a mass of 64.93 amu. from the atomic weight of cu = 63.54 one can conclude that:
- both isotopes have the same percent natural abundance
- copper-65 has the highest percent natural abundance
- most copper atoms have an atomic mass of 63.54
- copper-63 has the highest percent natural abundance

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1


Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
The element copper has two stable isotopes, copper-63 with a mass of 62.93 amu and copper-65 with a...
Questions


Chemistry, 20.07.2019 14:00

English, 20.07.2019 14:00

Mathematics, 20.07.2019 14:00

Spanish, 20.07.2019 14:00

Geography, 20.07.2019 14:00

Mathematics, 20.07.2019 14:00


Health, 20.07.2019 14:00

History, 20.07.2019 14:00


Mathematics, 20.07.2019 14:00






Spanish, 20.07.2019 14:00