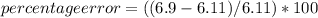Chemistry, 24.01.2020 00:31 GhostBoooty
In a laboratory activity, the density of a sample of vanadium is determined to be 6.9 g/cm3 at room temperature. what is the percent error for the determined value?
a. 0.15%
b. 0.87%
c. 13%
d. 15%

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1


Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1

Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1
You know the right answer?
In a laboratory activity, the density of a sample of vanadium is determined to be 6.9 g/cm3 at room...
Questions





Social Studies, 03.07.2019 17:00

History, 03.07.2019 17:00

History, 03.07.2019 17:00

History, 03.07.2019 17:00

History, 03.07.2019 17:00

Social Studies, 03.07.2019 17:00

History, 03.07.2019 17:00

History, 03.07.2019 17:00

Social Studies, 03.07.2019 17:00

Business, 03.07.2019 17:00

Biology, 03.07.2019 17:00


Biology, 03.07.2019 17:00


History, 03.07.2019 17:00