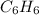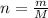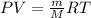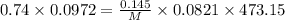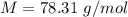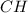Chemistry, 24.01.2020 00:31 makaylahunt
The empirical formula of a compound is ch. at 200 degree c, 0.145 g of this compound occupies 97.2 ml at a pressure of 0.74 atm. what is the molecular formula of the compound

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
The empirical formula of a compound is ch. at 200 degree c, 0.145 g of this compound occupies 97.2 m...
Questions


Mathematics, 31.10.2020 03:50


Mathematics, 31.10.2020 03:50

Social Studies, 31.10.2020 03:50

Chemistry, 31.10.2020 03:50

English, 31.10.2020 03:50

Biology, 31.10.2020 03:50



Social Studies, 31.10.2020 03:50



Mathematics, 31.10.2020 03:50

Mathematics, 31.10.2020 03:50

Mathematics, 31.10.2020 03:50