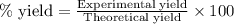During studies of the reaction below,
2 n2h4(l) + n2o4(l) ? 3 n2(g) + 4 h2o(g)
a chemical engineer measured a less-than-expected yield of n2 and discovered that the following side reaction occurs.
n2h4(l) + 2 n2o4(l) ? 6 no(g) + 2 h2o(g)
in one experiment, 11.5 g of no formed when 102.1 g of each reactant was used.
what is the highest percent yield of n2 that can be expected?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
During studies of the reaction below,
2 n2h4(l) + n2o4(l) ? 3 n2(g) + 4 h2o(g)
a chem...
2 n2h4(l) + n2o4(l) ? 3 n2(g) + 4 h2o(g)
a chem...
Questions


Computers and Technology, 17.09.2019 00:00









Computers and Technology, 17.09.2019 00:00









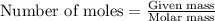 .....(1)
.....(1)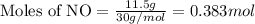
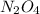 :
: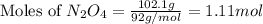
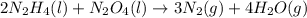 ......(2)
......(2)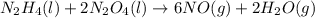 .......(3)
.......(3)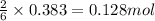 of
of 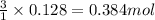 of nitrogen gas
of nitrogen gas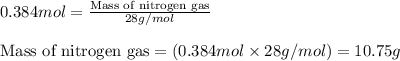
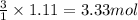 of nitrogen gas
of nitrogen gas