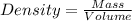To save time you can approximate the initial volume of water to ±1 ml and the initial mass of the solid to ±1 g. for example, if you are asked to add 23 ml of water, add between 22 ml and 24 ml. which metals in each of the following sets will have equal density?
1. 20.2 g gold placed in 21.6 ml of water and 12.0 g copper placed in 21.6 ml of water.
2. 20.2 g silver placed in 21.6 ml of water and 12.0 g silver placed in 21.6 ml of water.
3. 15.2 g copper placed in 21.6 ml of water and 50.0 g copper placed in 23.4 ml of water.
4. 15.4 g gold placed in 20.0 ml of water and 15.7 g silver placed in 20.0 ml of water.
5. 20.2 g silver placed in 21.6 ml of water and 20.2 g copper placed in 21.6 ml of water.
6. 11.2 g gold placed in 21.6 ml of water and 14.9 g gold placed in 23.4 ml of water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
To save time you can approximate the initial volume of water to ±1 ml and the initial mass of the so...
Questions


Chemistry, 17.05.2021 01:00


Social Studies, 17.05.2021 01:00

Mathematics, 17.05.2021 01:00


History, 17.05.2021 01:00


Mathematics, 17.05.2021 01:00

Biology, 17.05.2021 01:00


Physics, 17.05.2021 01:00

Mathematics, 17.05.2021 01:00


Computers and Technology, 17.05.2021 01:00

English, 17.05.2021 01:00

English, 17.05.2021 01:00

Mathematics, 17.05.2021 01:00

Mathematics, 17.05.2021 01:00