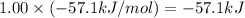Hcl(aq)+naoh(aq)→nacl(aq)+h2o(l)δh° =−57.1kj/molrxn
the chemical equation above represents th...

Chemistry, 23.01.2020 20:31 powellkolbie
Hcl(aq)+naoh(aq)→nacl(aq)+h2o(l)δh° =−57.1kj/molrxn
the chemical equation above represents the reaction between hcl(aq) and naoh(aq). when equal volumes of 1.00mhcl(aq) and 1.00mnaoh(aq) are mixed, 57.1kj of heat is released. if the experiment is repeated with 2.00mhcl(aq), how much heat would be released?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
Questions

Mathematics, 13.01.2020 15:31

English, 13.01.2020 15:31

Mathematics, 13.01.2020 15:31

Physics, 13.01.2020 16:31

Social Studies, 13.01.2020 16:31




History, 13.01.2020 16:31


Biology, 13.01.2020 16:31



Arts, 13.01.2020 16:31


Mathematics, 13.01.2020 16:31

Chemistry, 13.01.2020 16:31

English, 13.01.2020 16:31


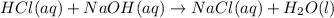 ΔH°=-57.1kJ/mol
ΔH°=-57.1kJ/mol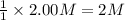 of NaOH
of NaOH