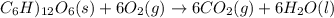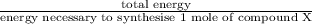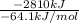Chemistry, 23.01.2020 03:31 coleman310
Assume that the complete combustion of one mole of fructose, a monosaccharide, to carbon dioxide and water liberates 2810 kj (δg°\' = –2810 kj/mol). if the energy generated by the combustion of fructose is entirely converted to the synthesis of a hypothetical compound x, calculate the number of moles of the compound that could theoretically be generated. use the value δg°\'compound x = − 64.1 kj/mol kj/mol. round your answer to two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2


Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2
You know the right answer?
Assume that the complete combustion of one mole of fructose, a monosaccharide, to carbon dioxide and...
Questions



English, 03.09.2020 06:01

Geography, 03.09.2020 06:01


Mathematics, 03.09.2020 06:01







Mathematics, 03.09.2020 06:01

Chemistry, 03.09.2020 06:01





Social Studies, 03.09.2020 06:01

Mathematics, 03.09.2020 06:01