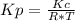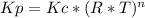Chemistry, 23.01.2020 03:31 bakaoffire
Consider the following chemical equilibrium: c(s) +2h2 (g) ch4 (g) now write an equation below that shows how to calculate from for this reaction at an absolute temperature . you can assume is comfortably above room temperature. if you include any common physical constants in your equation be sure you use their standard symbols, found in the aleks calculator.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1
You know the right answer?
Consider the following chemical equilibrium: c(s) +2h2 (g) ch4 (g) now write an equation below that...
Questions






Mathematics, 27.07.2020 01:01

Mathematics, 27.07.2020 01:01

Mathematics, 27.07.2020 01:01

Geography, 27.07.2020 01:01

Biology, 27.07.2020 01:01

Mathematics, 27.07.2020 01:01

Mathematics, 27.07.2020 01:01





Mathematics, 27.07.2020 01:01


Geography, 27.07.2020 01:01

Mathematics, 27.07.2020 01:01