
Chemistry, 23.01.2020 03:31 diamondpositive68
Amixture of xenon and oxygen gas is compressed from a volume of 99.0 l to a volume of 98.0 l, while the pressure is held constant at 69.0 atm. calculate the work done on the gas mixture. be sure your answer has the correct sign (positive or negative) and the correct number of significant digits

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1


You know the right answer?
Amixture of xenon and oxygen gas is compressed from a volume of 99.0 l to a volume of 98.0 l, while...
Questions

Computers and Technology, 08.05.2021 14:40

Biology, 08.05.2021 14:40

History, 08.05.2021 14:40

Mathematics, 08.05.2021 14:40

English, 08.05.2021 14:40

English, 08.05.2021 14:40


Law, 08.05.2021 14:40

English, 08.05.2021 14:40

Chemistry, 08.05.2021 14:40



Mathematics, 08.05.2021 14:40

Biology, 08.05.2021 14:40


Chemistry, 08.05.2021 14:40

Chemistry, 08.05.2021 14:40

English, 08.05.2021 14:40

Mathematics, 08.05.2021 14:40

English, 08.05.2021 14:40

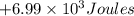
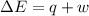
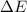 =Change in internal energy
=Change in internal energy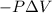 {Work done on the system is positive when the final volume is lesser than initial volume }
{Work done on the system is positive when the final volume is lesser than initial volume }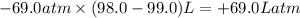
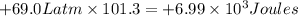 {1Latm=101.3J}
{1Latm=101.3J}

