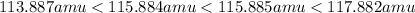The two naturally occurring isotopes of bromine are
81br (80.916 amu, 49.31%) and
79br (78.918 amu, 50.69%).
the two naturally occurring isotopes of chlorine are
37cl (36.966 amu, 24.23%) and
35cl (34.969 amu, 75.77%).
bromine and chlorine combine to form bromine monochloride, brcl.
what are the masses of the four different brcl molecules? express the masses in atomic mass units using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

You know the right answer?
The two naturally occurring isotopes of bromine are
81br (80.916 amu, 49.31%) and
<...
81br (80.916 amu, 49.31%) and
<...
Questions



Mathematics, 16.05.2021 19:20



Mathematics, 16.05.2021 19:20

Mathematics, 16.05.2021 19:20




Computers and Technology, 16.05.2021 19:20


Mathematics, 16.05.2021 19:20




History, 16.05.2021 19:20


Mathematics, 16.05.2021 19:20

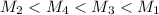
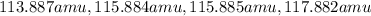
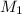 = 80.916 amu + 36.966 amu
= 80.916 amu + 36.966 amu 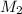 = 78.918 amu + 34.969 amu
= 78.918 amu + 34.969 amu 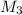 = 80.916 amu + 34.969 amu
= 80.916 amu + 34.969 amu 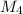 = 78.918 amu + 36.966 amu
= 78.918 amu + 36.966 amu