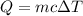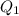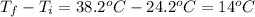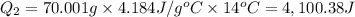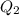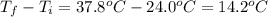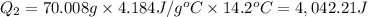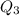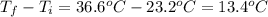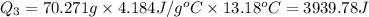Scoring scheme: 3-3-2-1 part i. for each trial, enter the amount of heat gained by the cool water, qcool water. the specific heat of water is 4.184 j/goc. report your answer to 4 digits. note: you should always carry 1 or 2 extra digits beyond the number of significant figures until your final calculation.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
Scoring scheme: 3-3-2-1 part i. for each trial, enter the amount of heat gained by the cool water,...
Questions


Geography, 26.07.2019 18:30

Mathematics, 26.07.2019 18:30

English, 26.07.2019 18:30


Biology, 26.07.2019 18:30

Biology, 26.07.2019 18:30

Physics, 26.07.2019 18:30

History, 26.07.2019 18:30

Biology, 26.07.2019 18:30

History, 26.07.2019 18:30

Biology, 26.07.2019 18:30




Physics, 26.07.2019 18:30


Physics, 26.07.2019 18:30


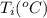 24.2, 24.0 , 23.2
24.2, 24.0 , 23.2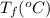 38.2, 37.8 , 36.6
38.2, 37.8 , 36.6