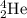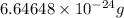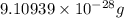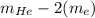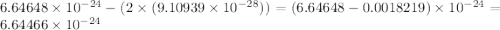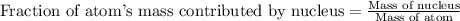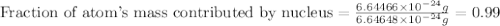Helium is the lightest noble gas and the second most abundant element (after hydrogen) in the universe. the mass of a helium−4 atom is 6.64648 × 10−24g, and each of its two electrons has a mass of 9.10939 × 10−28g. what fraction of this atom's mass is contributed by its nucleus?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
Helium is the lightest noble gas and the second most abundant element (after hydrogen) in the univer...
Questions




Mathematics, 11.07.2019 15:00

Arts, 11.07.2019 15:00


History, 11.07.2019 15:00


History, 11.07.2019 15:00

English, 11.07.2019 15:00


Mathematics, 11.07.2019 15:00






Chemistry, 11.07.2019 15:00