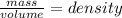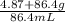Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1


Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
Asolution is made by dissolving 4.87 g of potassium nitrate in water to a final volume of 86.4 ml so...
Questions



Mathematics, 29.08.2020 21:01


Mathematics, 29.08.2020 21:01

Mathematics, 29.08.2020 21:01



Social Studies, 29.08.2020 21:01

Business, 29.08.2020 21:01





Business, 29.08.2020 21:01

World Languages, 29.08.2020 21:01

Chemistry, 29.08.2020 21:01



Advanced Placement (AP), 29.08.2020 21:01