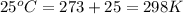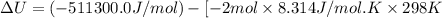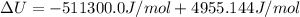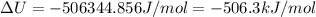Chemistry, 22.01.2020 04:31 george6871
The standard internal energy change for a reaction can be symbolized as δ u ∘ rxn or δ e ∘ rxn . for each reaction equation, calculate the energy change of the reaction at 25 ∘ c and 1.00 bar . sn ( s ) + 2 cl 2 ( g ) ⟶ sncl 4 ( l ) δ h ∘ rxn = − 511.3 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

You know the right answer?
The standard internal energy change for a reaction can be symbolized as δ u ∘ rxn or δ e ∘ rxn . for...
Questions

Mathematics, 13.01.2020 05:31

Biology, 13.01.2020 05:31

Biology, 13.01.2020 05:31


Mathematics, 13.01.2020 05:31


Mathematics, 13.01.2020 05:31



Mathematics, 13.01.2020 05:31


English, 13.01.2020 05:31

Mathematics, 13.01.2020 05:31

Mathematics, 13.01.2020 05:31




History, 13.01.2020 05:31


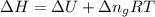
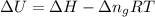
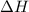 = change in enthalpy =
= change in enthalpy = 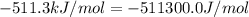
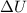 = change in internal energy = ?
= change in internal energy = ?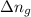 = change in moles
= change in moles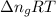 = 0
= 0