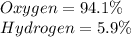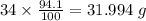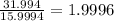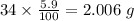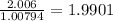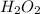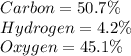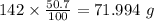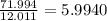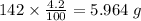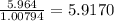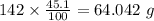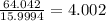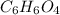The molecular formula for compound (a) is H₂O₂
The molecular formula for compound (a) is C₆H₆O₄
Explanation:
A compound's molar mass determines what is the mass of one mole of that substance. One mole of a compound contains Avogadro's number of molecules =  molecules.
molecules.
The percent composition of elements in 1 mole of compound can be used to get the exact number of moles of each element present in per mole of compound.
................................................................................................................................................
a) 94.1% Oxygen and 5.9% Hydrogen; molar mass = 34 g
The given compound's percent composition is as follows
:
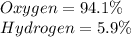
For O :
94.1% of oxygen refers to 94.1 g of Oxygen per 100 g of compound.
Mass of Oxygen in 34 g of compound = 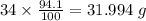
To determine the number of moles of element O present in one mole of compound
:
Molar mass of Oxygen = 15.9994 g/mol
The mass in grams of one mole of substance is called molar mass.
One mole of oxygen has 15.9994 grams of O.
Number of moles of O in 31.994 g of O = 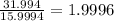
In whole numbers,
Number of moles of O in 31.994 g of compound = 1.9996 ≈ 2
Similarly,
For H :
5.9% of hydrogen refers to 5.9 g of Hydrogen per 100 g of compound.
Mass of Hydrogen in 34 g of compound = 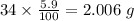
To determine the number of moles of element H present in one mole of compound
:
Molar mass of Hydrogen = 1.00794 g/mol
The mass in grams of one mole of substance is called molar mass.
One mole of hydrogen has 1.00794 grams of H.
Number of moles of H in 2.006 g of H = 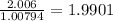
In whole numbers,
Number of moles of H in 2.006 g of compound = 1.9901 ≈ 2
The molecular formula for this compound (a) can be written as 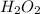
...........................................................................................................................................
b) 50.7% Carbon, 4.2% Hydrogen, and 45.1% Oxygen; molar mass = 142 g
The given compound's percent composition is as follows
:
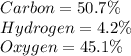
For C :
50.7% of carbon refers to 50.7 g of carbon per 100 g of compound.
Mass of Carbon in 142 g of compound = 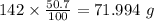
To determine the number of moles of element C present in one mole of compound
:
Molar mass of Carbon = 12.011 g/mol
The mass in grams of one mole of substance is called molar mass.
One mole of carbon has 12.011 grams of C.
Number of moles of C in 71.994 g of C = 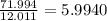
In whole numbers,
Number of moles of C in 71.994 g of compound = 5.9940 ≈ 6
For H :
4.2% of hydrogen refers to 4.2 g of Hydrogen per 100 g of compound.
Mass of Hydrogen in 142 g of compound = 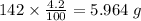
To determine the number of moles of element H present in one mole of compound
:
Molar mass of Hydrogen = 1.00794 g/mol
The mass in grams of one mole of substance is called molar mass.
One mole of hydrogen has 1.00794 grams of H.
Number of moles of H in 5.964 g of H = 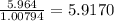
In whole numbers,
Number of moles of H in 2.006 g of compound = 5.9170 ≈ 6
For O :
45.1% of oxygen refers to 45.1 g of Oxygen per 100 g of compound.
Mass of Oxygen in 142 g of compound = 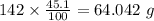
To determine the number of moles of element O present in one mole of compound
:
Molar mass of Oxygen = 15.9994 g/mol
The mass in grams of one mole of substance is called molar mass.
One mole of oxygen has 15.9994 grams of O.
Number of moles of O in 64.042 g of O = 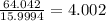
In whole numbers,
Number of moles of O in 31.994 g of compound = 4.002 ≈ 4
The molecular formula for this compound (b) can be written as 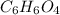




























 molecules.
molecules.