
Chemistry, 21.01.2020 22:31 edgartorres5123
According to a label on a bottle of concentrated hydrochloric acid, the contents are 36.0% hcl by mass and have a density of 1.18 g/ml.
1. what is the molarity of concentrated hcl.
2. what volume of it would you need to prepare 985 ml of 1.6 m hcl?
3. what mass of sodium bicarbonate would be needed to neutralize the spill if a bottle containing 1.75 l of concentrated hcl dropped on a lab floor and broke open?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
According to a label on a bottle of concentrated hydrochloric acid, the contents are 36.0% hcl by ma...
Questions




Mathematics, 09.01.2021 17:30


Geography, 09.01.2021 17:30

Biology, 09.01.2021 17:30


Computers and Technology, 09.01.2021 17:30

Social Studies, 09.01.2021 17:30




Medicine, 09.01.2021 17:30




Health, 09.01.2021 17:30


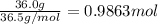
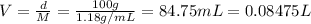
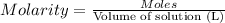
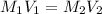 ( Dilution equation)
( Dilution equation)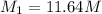
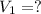
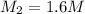
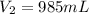
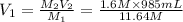
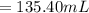
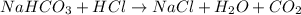
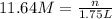
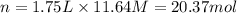
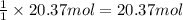 of sodium carbonate
of sodium carbonate 

