
A= ε l c. (a) define the terms in the formula: a = ε l c. (pick your answers using the letter of the correct definition.)
a. pathlength of light through the cell
b. concentration, in mol/l, of the stock solution from which the sample was made
c. absorbance measured by the spectrometer
d. molar absorptivity, a constant unique to that substance at that wavelength
e. the wavelength of the light being used for the measurement
f. concentration, in mol/l, of the sample solution being measured definition of l definition of c definition of ε definition of a

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1

Chemistry, 23.06.2019 09:10
In a 28 g serving of cheese curls there are 247mg of sodium. how much sodium is in a 12.5 ounce bag
Answers: 1
You know the right answer?
A= ε l c. (a) define the terms in the formula: a = ε l c. (pick your answers using the letter of th...
Questions



Geography, 11.06.2020 21:57

Mathematics, 11.06.2020 21:57



English, 11.06.2020 21:57




Biology, 11.06.2020 21:57

Mathematics, 11.06.2020 21:57


Mathematics, 11.06.2020 21:57

Social Studies, 11.06.2020 21:57


English, 11.06.2020 21:57

Mathematics, 11.06.2020 21:57

Mathematics, 11.06.2020 21:57

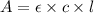
 = molar absorptivity of this solution
= molar absorptivity of this solution

