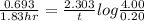Fluorine-18 (18f) is a radioactive isotope use in a variety of medical imaging procedures including positron emission tomography (pet) scans.
fluorine-18 decays by positron emission with a half-life of 1.83 hours.
(1) when 18f undergoes positron emission, the product nucleus is,
a) 18o b) 19ne c) 14n d) 17f
(2) a typical dose of 18f used for a pet scan has an activity of 4.00 millicuries. how long will it take for 95% of the 18f to decay?
a) 1.74 hrs b) 5.49 min c) 8.13 minutes d) 7.91 hrs.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1


Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
Fluorine-18 (18f) is a radioactive isotope use in a variety of medical imaging procedures including...
Questions

English, 11.07.2019 02:30




Health, 11.07.2019 02:30

History, 11.07.2019 02:30








Mathematics, 11.07.2019 02:30

Mathematics, 11.07.2019 02:30

Computers and Technology, 11.07.2019 02:30


Chemistry, 11.07.2019 02:30


 . Therefore, when a positron emission occurs then the resultant nuclei atomic number decreases by a unit mass.
. Therefore, when a positron emission occurs then the resultant nuclei atomic number decreases by a unit mass. 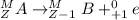
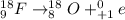
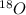 .
.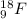 isotope is as follows.
isotope is as follows.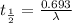

![[N]_{o}](/tpl/images/0463/6241/b711d.png) ) = 4.00 millicuries
) = 4.00 millicuries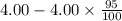
![\lambda = \frac{2.303}{t} log \frac{[N]_{o}}{[N]_{t}}](/tpl/images/0463/6241/b899d.png)