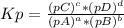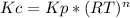Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1


Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Ammonium hydrosulfide decomposes to ammonia and hydrogen sulfide gases. find kc for the decompositio...
Questions





Social Studies, 02.04.2020 02:08


History, 02.04.2020 02:08

History, 02.04.2020 02:08



Mathematics, 02.04.2020 02:08

Mathematics, 02.04.2020 02:08

Social Studies, 02.04.2020 02:08

Mathematics, 02.04.2020 02:08


Mathematics, 02.04.2020 02:08

Mathematics, 02.04.2020 02:08



![Kc = \frac{[C]^c*[D]^d}{[A]^a*[B]^b}](/tpl/images/0463/5599/833e7.png)