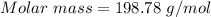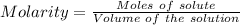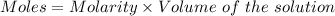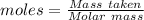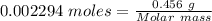Chemistry, 21.01.2020 03:31 Peachyyyyyy978
0.456 grams of a monoprotic acid is titrated with 45.88 ml of 0.0500 m naoh. what is the molecular mass (molar mass) of the acid

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2
You know the right answer?
0.456 grams of a monoprotic acid is titrated with 45.88 ml of 0.0500 m naoh. what is the molecular m...
Questions


History, 05.11.2020 08:40


English, 05.11.2020 08:40

Biology, 05.11.2020 08:40


German, 05.11.2020 08:40

History, 05.11.2020 08:40

World Languages, 05.11.2020 08:40

Mathematics, 05.11.2020 08:40



English, 05.11.2020 08:40

Mathematics, 05.11.2020 08:40


Social Studies, 05.11.2020 08:40

History, 05.11.2020 08:40

English, 05.11.2020 08:40