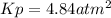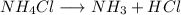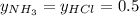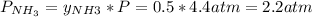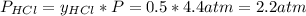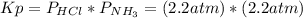Chemistry, 21.01.2020 01:31 mslamaya11
Asample of solid ammonium chloride was placed into an evacuated rigid container and then heated so that it decomposed to ammonia gas and hydrogen chloride gas. after heating the total pressure in the container was found to be 4.4 atm. calculate kp at this temperature for the decomposition reaction

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
Asample of solid ammonium chloride was placed into an evacuated rigid container and then heated so t...
Questions

English, 27.09.2019 15:10

Biology, 27.09.2019 15:10

Geography, 27.09.2019 15:10




Geography, 27.09.2019 15:10

Mathematics, 27.09.2019 15:10

Social Studies, 27.09.2019 15:10

History, 27.09.2019 15:10

Mathematics, 27.09.2019 15:10

Chemistry, 27.09.2019 15:20

Mathematics, 27.09.2019 15:20

History, 27.09.2019 15:20



Spanish, 27.09.2019 15:20

History, 27.09.2019 15:20


Mathematics, 27.09.2019 15:20