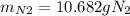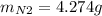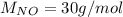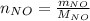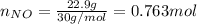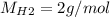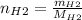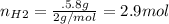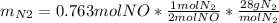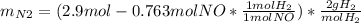Chemistry, 20.01.2020 23:31 jewlbug4358
For the following reaction, 22.9 grams of nitrogen monoxide are allowed to react with 5.80 grams of hydrogen gas. nitrogen monoxide (g) + hydrogen (g)> nitrogen (g) + water (1) what is the maximum amount of nitrogen gas that can be formed? what is the formula for the limiting reagent? grams what amount of the excess reagent remains after the reaction is complete? grams

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1
You know the right answer?
For the following reaction, 22.9 grams of nitrogen monoxide are allowed to react with 5.80 grams of...
Questions


Mathematics, 18.12.2019 07:31

Mathematics, 18.12.2019 07:31

English, 18.12.2019 07:31



Chemistry, 18.12.2019 07:31

Physics, 18.12.2019 07:31


History, 18.12.2019 07:31

Mathematics, 18.12.2019 07:31

Biology, 18.12.2019 07:31



Mathematics, 18.12.2019 07:31

History, 18.12.2019 07:31

Mathematics, 18.12.2019 07:31

History, 18.12.2019 07:31