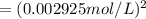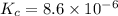Chemistry, 20.01.2020 21:31 amandanunnery33
Titanium(iv) chloride decomposes to form titanium and chlorine, like this: (l)(s)(g) at a certain temperature, a chemist finds that a reaction vessel containing a mixture of titanium(iv) chloride, titanium, and chlorine at equilibrium has the following composition: compound amount calculate the value of the equilibrium constant for this reaction. round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1
You know the right answer?
Titanium(iv) chloride decomposes to form titanium and chlorine, like this: (l)(s)(g) at a certain t...
Questions

English, 26.04.2021 23:10



English, 26.04.2021 23:10


Mathematics, 26.04.2021 23:10

Computers and Technology, 26.04.2021 23:10


Mathematics, 26.04.2021 23:10

Mathematics, 26.04.2021 23:10

Mathematics, 26.04.2021 23:10

Health, 26.04.2021 23:10

Mathematics, 26.04.2021 23:10

Mathematics, 26.04.2021 23:10

Mathematics, 26.04.2021 23:10

Mathematics, 26.04.2021 23:10



Mathematics, 26.04.2021 23:10

Mathematics, 26.04.2021 23:10

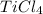 4.18 g
4.18 g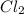 1.08g
1.08g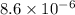 the value of the equilibrium constant for this reaction.
the value of the equilibrium constant for this reaction.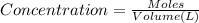
![[TiCl_4]=\frac{4.18 g}{190 g/mol\times 5.2 L}=0.004231 mol/L](/tpl/images/0463/1030/e0fe7.png)
![[Ti]=\frac{1.32 g}{48 g/mol\times 5.2 L}=0.005288 mol/L](/tpl/images/0463/1030/9e0bf.png)
![[Cl_2]=\frac{1.08 g}{71 g/mol\times 5.2 L}=0.002925 mol/L](/tpl/images/0463/1030/2b02a.png)
![K_c=[Cl_2]^2](/tpl/images/0463/1030/ae7ed.png)