Chemistry, 25.12.2019 00:31 valenciafaithtorres
Consider the following reaction: . so2cl2(g)⇌so2(g)+cl2(g) . a reaction mixture is made containing an initial [so2cl2] of 2.2×10−2m . at equilibrium, [cl2]= 1.3×10−2m .. calculate the value of the equilibrium constant (kc).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3
You know the right answer?
Consider the following reaction: . so2cl2(g)⇌so2(g)+cl2(g) . a reaction mixture is made containing...
Questions



Social Studies, 30.08.2019 17:30

Biology, 30.08.2019 17:30


Mathematics, 30.08.2019 17:30

Mathematics, 30.08.2019 17:30









Biology, 30.08.2019 17:30


History, 30.08.2019 17:30

Physics, 30.08.2019 17:30

Mathematics, 30.08.2019 17:30

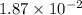
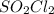 =
= 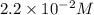
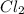 =
= 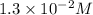
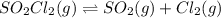

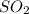 =
= ![K_c=\frac{[SO_2]\times [Cl_2]}{[SO_2Cl_2]}](/tpl/images/0432/3201/33429.png)