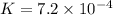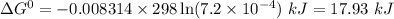Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2

Chemistry, 23.06.2019 06:30
Which of these natural resources is non-renewable a.corn b.wind c.geothermal d.natural gas
Answers: 2
You know the right answer?
Calculate δg° for the following reaction from the equilibrium constant at the temperature given. hf(...
Questions

Biology, 12.06.2020 21:57



English, 12.06.2020 21:57

Mathematics, 12.06.2020 21:57




English, 12.06.2020 21:57



Mathematics, 12.06.2020 21:57




Mathematics, 12.06.2020 21:57




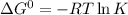
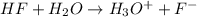
![25^oC=[25+273]K=298K](/tpl/images/0462/9739/df1f6.png)