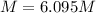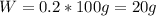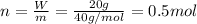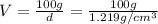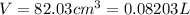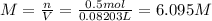Chemistry, 20.01.2020 20:31 madams4450
A20.0%(m/m) solution of naoh (fm 40.00) has a density of 1.219 g/cm'. calculate the solution's molarity. (6.10 m) 2. * 10 w= amountiof solute d- density of solution motanty m= molecular mass ot solute %3d

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
A20.0%(m/m) solution of naoh (fm 40.00) has a density of 1.219 g/cm'. calculate the solution's molar...
Questions

History, 25.09.2019 15:10

Geography, 25.09.2019 15:10



Chemistry, 25.09.2019 15:10

Mathematics, 25.09.2019 15:10

Biology, 25.09.2019 15:10

English, 25.09.2019 15:10


Mathematics, 25.09.2019 15:10




Mathematics, 25.09.2019 15:10


History, 25.09.2019 15:10