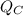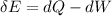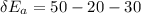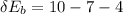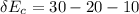Chemistry, 20.01.2020 19:31 babyduckies37
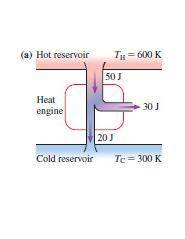
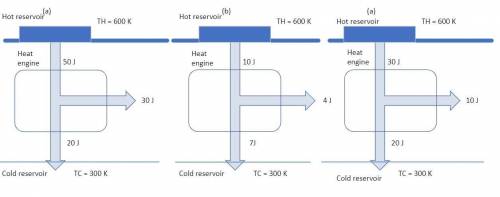
For each engine calculate δe = qh−wout−qc, where qh is the amount of heat transferred from the hot reservoir, qc is the amount of heat transferred to the cold reservoir, wout is the energy output of the heat engine. qh, wout, qc are positive quantities.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
For each engine calculate δe = qh−wout−qc, where qh is the amount of heat transferred from the hot r...
Questions

History, 17.02.2021 20:00


Mathematics, 17.02.2021 20:00


English, 17.02.2021 20:00

Mathematics, 17.02.2021 20:00

Biology, 17.02.2021 20:00


Mathematics, 17.02.2021 20:00

History, 17.02.2021 20:00


History, 17.02.2021 20:00

Mathematics, 17.02.2021 20:00


Mathematics, 17.02.2021 20:00

English, 17.02.2021 20:00

Mathematics, 17.02.2021 20:00

English, 17.02.2021 20:00


Mathematics, 17.02.2021 20:00

 -
- 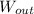 -
-