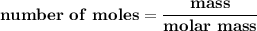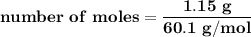Suppose that 1.15 g of rubbing alcohol (c3h8o) evaporates from a 65.0-g aluminum block. if the aluminum block is initially at 25 °c, what is the final temperature of the block after the evapo- ration of the alcohol? assume that the heat required for the vaporization of the alcohol comes only from the aluminum block and that the alcohol vaporizes at 25 °c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
Suppose that 1.15 g of rubbing alcohol (c3h8o) evaporates from a 65.0-g aluminum block. if the alumi...
Questions

Mathematics, 29.08.2019 04:30

Mathematics, 29.08.2019 04:30

Mathematics, 29.08.2019 04:30


Business, 29.08.2019 04:30

Mathematics, 29.08.2019 04:30

Spanish, 29.08.2019 04:30

Biology, 29.08.2019 04:30


Business, 29.08.2019 04:30



Mathematics, 29.08.2019 04:30




Chemistry, 29.08.2019 04:30


Mathematics, 29.08.2019 04:30