The following reaction is done at
t = 25°c and p= 1.0 atm:
ca (s) + 2 hcl (aq) → c...

Chemistry, 20.01.2020 03:31 tatianaflores9040
The following reaction is done at
t = 25°c and p= 1.0 atm:
ca (s) + 2 hcl (aq) → cacl_2 (aq) + h_2 (g)
if 500.0 g of calcium are added to an excess of hci, what volume of h_2 is produced?
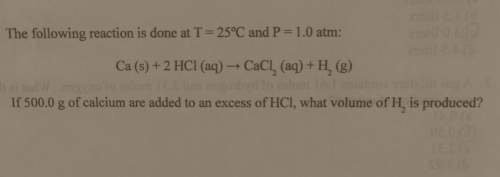

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

You know the right answer?
Questions

Computers and Technology, 22.10.2021 22:20

Biology, 22.10.2021 22:20


Social Studies, 22.10.2021 22:20

Social Studies, 22.10.2021 22:20






Computers and Technology, 22.10.2021 22:20


Physics, 22.10.2021 22:20

Mathematics, 22.10.2021 22:20

English, 22.10.2021 22:20

History, 22.10.2021 22:20

Chemistry, 22.10.2021 22:20


English, 22.10.2021 22:20

Advanced Placement (AP), 22.10.2021 22:20




