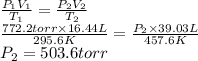Chemistry, 18.01.2020 06:31 joshlynn52
Acylinder fitted with a movable piston and filled with a gas has a volume of 16.44 liters at 22.4°c when the applied pressure is 772.2 torr.
the temperature of the oil bath surrounding the piston was increased to 184.4°c, and the pressure on the piston was changed. careful measurement now gave a value of 39.03 liters for the new volume.
what is the final pressure in the cylinder, expressed in torr?
a) 494.0 torr
b) 503.6 torr
c) 1184 torr
d) 1207 torr
e) 6295 torr
with explanation .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 23.06.2019 06:30
Generally, observed behavior that can be formulated into a statement, sometimes mathematical in nature, is called a(n): a. observation. b. measurement. c. theory. d. natural law. e. experiment.
Answers: 2

Chemistry, 23.06.2019 13:30
1. what is boyle’s law? • state the definition of the law in words. • what are the assumptions of boyle’s law? • write at least one mathematical equation that represents the law. • what can be calculated with boyle’s law? • using a gas-filled balloon as an example, describe what is happening to the gas molecules inside the balloon before and after you squeeze it.
Answers: 2
You know the right answer?
Acylinder fitted with a movable piston and filled with a gas has a volume of 16.44 liters at 22.4°c...
Questions

Mathematics, 01.01.2021 07:40




Computers and Technology, 01.01.2021 07:40

Social Studies, 01.01.2021 07:40

Computers and Technology, 01.01.2021 07:40


Mathematics, 01.01.2021 07:40

Advanced Placement (AP), 01.01.2021 07:40


Mathematics, 01.01.2021 07:40

English, 01.01.2021 07:40


English, 01.01.2021 07:40

Mathematics, 01.01.2021 07:40


Biology, 01.01.2021 07:50

Physics, 01.01.2021 07:50

English, 01.01.2021 07:50