

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1


Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
Which type of orbital will be occupied by the electrons of highest energy for the si^4+ ground-state...
Questions




Computers and Technology, 13.10.2021 01:30

World Languages, 13.10.2021 01:30

Biology, 13.10.2021 01:30


Biology, 13.10.2021 01:30

Business, 13.10.2021 01:30


Arts, 13.10.2021 01:30

Chemistry, 13.10.2021 01:30

Mathematics, 13.10.2021 01:30



Mathematics, 13.10.2021 01:30




English, 13.10.2021 01:30

![[Ne]3s^{2}3p^{2}](/tpl/images/0460/3364/54c8d.png) .
. 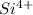 has a +4 charge which means that it has lost 4 electrons. Hence, the electronic configuration of
has a +4 charge which means that it has lost 4 electrons. Hence, the electronic configuration of 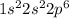 .
. 

