

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1


Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
For h3po4, ka1 = 7.3 x 10^-3, ka2 = 6.2 x 10^-6, and ka3 = 4.8 x 10^-13. a 0.10 m aqueous solution o...
Questions

Social Studies, 16.04.2020 00:31

History, 16.04.2020 00:31


Mathematics, 16.04.2020 00:31



Biology, 16.04.2020 00:31





Health, 16.04.2020 00:31

English, 16.04.2020 00:31





Mathematics, 16.04.2020 00:31

Mathematics, 16.04.2020 00:31

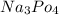 therefore would be "strongly basic."
therefore would be "strongly basic."

