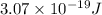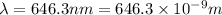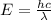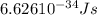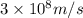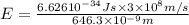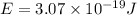When an electron in a 2p orbital of a particular atom makes a transition to the 2s orbital, a photon of approximate wavelength 646.3 nm is emitted. the energy difference between these 2p and 2s orbitals is: . 3.07 ã 10^â28 jb. 3.07 ã 10^â19 jc. 3.07 ã 10^â17 jd. 1.28 ã 10^â31 je. none of these

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3
You know the right answer?
When an electron in a 2p orbital of a particular atom makes a transition to the 2s orbital, a photon...
Questions











Mathematics, 31.03.2020 03:59

Business, 31.03.2020 03:59