
Chemistry, 18.01.2020 00:31 gabriella1232002
Raising the temperature of 10.0 g of water from 10.0°c to 20.0°c requires 100.0 cal of energy, while raising the temperature of 10.0 g of aluminum from 10.0°c to 20.0°c requires 22 cal. more calories are required to heat the water

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
Raising the temperature of 10.0 g of water from 10.0°c to 20.0°c requires 100.0 cal of energy, while...
Questions


Biology, 04.10.2020 06:01

Mathematics, 04.10.2020 06:01

Mathematics, 04.10.2020 06:01


Mathematics, 04.10.2020 06:01

Mathematics, 04.10.2020 06:01


Physics, 04.10.2020 06:01


Chemistry, 04.10.2020 06:01

Chemistry, 04.10.2020 06:01


Biology, 04.10.2020 06:01

English, 04.10.2020 06:01

Mathematics, 04.10.2020 06:01

Biology, 04.10.2020 06:01



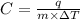
 = change in temperature of substance
= change in temperature of substance

