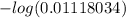Chemistry, 18.01.2020 00:31 jaquisjones68
Farmers who raise cotton once used arsenic acid, h₃aso₄, as a defoliant at harvest time. arsenic acid is a polyprotic acid with ka₁ = 2.5 × 10⁻⁴, ka₂ = 5.6 × 10⁻⁸, and ka₃ = 3 × 10⁻¹³. what is the ph of a 0.500 m solution of arsenic acid?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2


Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2
You know the right answer?
Farmers who raise cotton once used arsenic acid, h₃aso₄, as a defoliant at harvest time. arsenic aci...
Questions

History, 01.02.2021 20:00



History, 01.02.2021 20:00



Chemistry, 01.02.2021 20:00




History, 01.02.2021 20:00


History, 01.02.2021 20:00



Mathematics, 01.02.2021 20:00


Mathematics, 01.02.2021 20:00

Mathematics, 01.02.2021 20:00

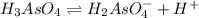
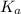 is as follows.
is as follows.![K_{a} = \frac{[H_{2}SO^{-}_{4}][H^{+}]}{[H_{3}AsO_{4}]}](/tpl/images/0460/0699/9663c.png)
![[H_{2}AsO^{-}_{4}]](/tpl/images/0460/0699/b1563.png) and
and ![[H^{+}]](/tpl/images/0460/0699/85507.png) is x.
is x.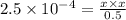
![-log [H^{+}]](/tpl/images/0460/0699/822be.png)