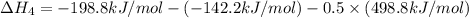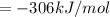Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2


Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
Using hess's law, what is îhâ°rxn for the following reaction? no(g) + o(g) â no2(g)no(g) + o3(g) â n...
Questions


History, 22.09.2019 18:50





Mathematics, 22.09.2019 18:50




Mathematics, 22.09.2019 18:50

Business, 22.09.2019 18:50

Mathematics, 22.09.2019 19:00

Mathematics, 22.09.2019 19:00






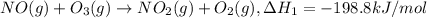 ..[1]
..[1]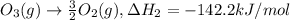 ..[2]
..[2]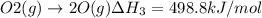 ..[3]
..[3]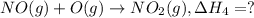 ..[4]
..[4]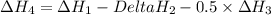 (By using Hess's law)
(By using Hess's law)