
The partial pressure of co2 gas in an unopened carbonated soft drink is 4.0 atm at 25°c. henry's law constant for co, in the soft drink is 3.1 x 102 mol/l atm at 25°c. the mole fraction of co2 in air at sea level is 3.3 x 104. a. what is the solubility of co2 gas-in-an-unopened soft drink bottle? nopened soft drink bottle? 3.1 x100/l x 4: 0 3 = 0.12 mol/l b. how many moles of co, gas are dissolved in an unopened 2 liter soft drink bottle? c. what is the solubility of co, gas in the opened soft drink at sea level? d. how many moles of co, gas are dissolved in 2 liters of soft drink left open at sea level?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1
You know the right answer?
The partial pressure of co2 gas in an unopened carbonated soft drink is 4.0 atm at 25°c. henry's law...
Questions


Mathematics, 13.05.2021 17:50


Mathematics, 13.05.2021 17:50

Arts, 13.05.2021 17:50

Mathematics, 13.05.2021 17:50

Biology, 13.05.2021 17:50

Mathematics, 13.05.2021 17:50

Biology, 13.05.2021 17:50




English, 13.05.2021 17:50

Social Studies, 13.05.2021 17:50


Mathematics, 13.05.2021 17:50



Mathematics, 13.05.2021 17:50

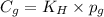
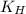 = Henry's constant
= Henry's constant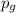 = partial pressure of gas
= partial pressure of gas 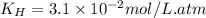
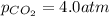
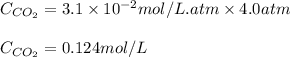
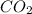 gas-in-an-unopened soft drink bottle.
gas-in-an-unopened soft drink bottle.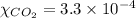
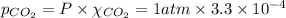
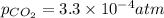
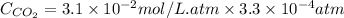
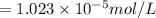
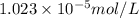 is the solubility of
is the solubility of 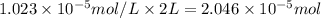
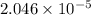 moles of [tex]CO_2[tex] gas are dissolved in 2 liter soft drink left open at sea level?
moles of [tex]CO_2[tex] gas are dissolved in 2 liter soft drink left open at sea level?

