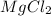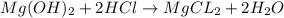Chemistry, 07.10.2019 17:50 aidanfbussiness
Check my semester exam ! i have a flight to must submit !
question 1 (multiple choice worth 2 points)
[07.06]what is the major difference between nuclear and chemical reactions?
only chemical reactions release energy.
only chemical reactions release gamma rays.
only nuclear reactions involve the exchange of electrons.
i picked: only nuclear reactions change the identity of an element.
question 2 (multiple choice worth 2 points)
[07.03]when water is added to an erlenmeyer flask and then sealed, phase equilibrium occurs. which answer explains the resulting dynamic equilibrium?
rate of evaporation = rate of precipitation
i picked: rate of condensation = rate of evaporation
moles of vapor = moles of liquid
moles of vapor = moles of solid
question 3 (multiple choice worth 2 points)
[05.01]which phase of matter is made up of particles that are packed closely together, with both a definite shape and a definite volume?
gas
liquid
plasma
i picked: solid
question 4 (multiple choice worth 2 points)
[06.02]when sulfuric acid is poured on sugar the volume of sugar expands and the sugar turns black and becomes very hot. which of the following best describes this reaction?
endothermic
i picked: exothermic
decomposition
synthesis
question 5 (multiple choice worth 2 points)
[07.01]which of the following is not true of acids?
acids are corrosive.
i picked: acids feel slippery.
acids have a sour taste.
acids have more hydronium ions than hydroxide ions.
question 6 (multiple choice worth 2 points)
[06.04]according to the collision theory, what is the best explanation for why a higher temperature makes a reaction go faster?
there are fewer collisions per minute.
it increases the distance between the particles.
it decreases the average kinetic energy of the particles.
i picked: it increases the average kinetic energy and number of collisions per minute.
question 7 (multiple choice worth 2 points)
[07.02]which of the following represents pure water?
[h3o+] > [oh-]
[h3o+] < [oh-]
i picked: [h3o+] = [oh-]
[h3o+] [oh-] = 14
question 8 (multiple choice worth 2 points)
[07.06]which of the following types of radiation can penetrate the most deeply into your body?
alpha rays
beta rays
i picked: gamma rays
proton rays
question 9 (multiple choice worth 2 points)
[07.05]in the chemical reaction shown below, which element was oxidized?
2k + cl2 yields 2kcl
i picked: k, because it lost one electron
k, because it gained one electron
cl, because it lost one electron
cl, because it gained one electron
question 10 (multiple choice worth 2 points)
[06.02]on an exothermic potential energy diagram, what is true for the products?
the products are at the same energy level as the reactants.
i picked: the products are at a lower energy level than the reactants.
the products are at a higher energy level than the reactants.
the products do not have energy.
question 11 (multiple choice worth 2 points)
[07.02]what is the salt formed in the following neutralization reaction?
mg(oh)2 + hcl yields h2o + mgcl2
mg(oh)2
hcl
h2o
i picked: mgcl2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 21:30
Electromagnets coils of wire paper clips picked up 10 3 15 6 20 9 25 12 ms. owens' class was studying magnets. ms. owens showed her students how to make an electromagnet using a nail, a d-cell battery, and plastic coated wire. the students wrapped the wire around the nail and then attached the ends to the battery. when they were finished, they tested their magnets by investigating how many paperclips their magnets could pick up. they also tested whether they could increase the strength of their electromagnets by using more coils of wire. they recorded the class average of their results in the data table seen here. ms. owens asked her students to graph their data in a line graph. how should the students label the x-axis on their line graph? a) size of battery b) number of paper clips c) number of coils of wire d) strength of electromagnet
Answers: 2


Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1
You know the right answer?
Check my semester exam ! i have a flight to must submit !
question 1 (multiple choice...
question 1 (multiple choice...
Questions

Spanish, 30.10.2020 19:30



Mathematics, 30.10.2020 19:30





English, 30.10.2020 19:30



Social Studies, 30.10.2020 19:30


Mathematics, 30.10.2020 19:30

English, 30.10.2020 19:30

Mathematics, 30.10.2020 19:30


Arts, 30.10.2020 19:30

History, 30.10.2020 19:30

Computers and Technology, 30.10.2020 19:30

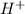 ion is their aqueous solution.They taste sour and corrosive in nature.
ion is their aqueous solution.They taste sour and corrosive in nature.![[H_3O^+]=[OH^-]](/tpl/images/0297/5506/f28a0.png)
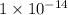
![pH=-\log[H_3O^+]=7](/tpl/images/0297/5506/4c7e1.png)
![[H_3O^+]=1\times 10^{-7}](/tpl/images/0297/5506/e8ae8.png)
![K_w=1\times 10^{-14}=[H_3O^+][OH^-]](/tpl/images/0297/5506/3cca3.png)