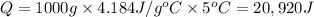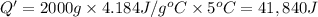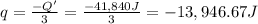Chemistry, 17.01.2020 06:31 dbn4everloved
A1.0 g sample of a cashew was burned in a calorimeter containing 1000. g of water, and the temperature of the water changed from 20.0°c to 25.0°c. in another experiment, a 3.0 g sample of a marshmallow was burned in a calorimeter containing 2000. g of water, and the temperature of the water changed from 25.0°c to 30.0°c. based on the data, which of the following can be concluded about the energy content for 1.0 g of each of the two substances? (the specific heat of water is 4.2 j/(g⋅° (a) the combustion of 1.0 g of cashew releases less energy than the combustion of 1.0 g of marshmallow. (b) the combustion of 1.0 g of cashew releases the same amount of energy as the^combustion of 1.0 g of marshmallow. (c) the combustion of 1.0 g of cashew releases more energy than the combustion of 1.0g of marshmallow. (d) no comparison can be made because the two systems started with different masses of food, different masses of water, and different initial temperatures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

You know the right answer?
A1.0 g sample of a cashew was burned in a calorimeter containing 1000. g of water, and the temperatu...
Questions

History, 12.09.2021 17:20




Social Studies, 12.09.2021 17:30

History, 12.09.2021 17:30


Social Studies, 12.09.2021 17:30

Biology, 12.09.2021 17:30


Biology, 12.09.2021 17:30



Computers and Technology, 12.09.2021 17:30


English, 12.09.2021 17:30


Mathematics, 12.09.2021 17:30

English, 12.09.2021 17:30

SAT, 12.09.2021 17:30