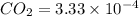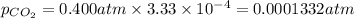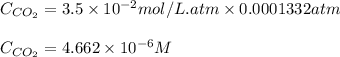Chemistry, 16.01.2020 22:31 deasiamonay14
Calculate the solubility of carbon dioxide in water at an atmospheric pressure of 0.400 atm (a typical value at high altitude).atmospheric gas mole fraction kh mol/(l*atm)n2 7.81 x 10-1 6.70 x 10-4o2 2.10 x 10-1 1.30 x 10-3ar 9.34 x 10-3 1.40 x 10-3co2 3.33 x 10-4 3.50 x 10-2ch4 2.00 x 10-6 1.40 x 10-3h2 5.00 x 10-7 7.80 x 10-4

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2


Chemistry, 23.06.2019 03:30
Ineed pls urgent 1-20 in order and fully detail step my step.
Answers: 1

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 1
You know the right answer?
Calculate the solubility of carbon dioxide in water at an atmospheric pressure of 0.400 atm (a typic...
Questions

Mathematics, 21.09.2019 01:00

History, 21.09.2019 01:00

English, 21.09.2019 01:00

Mathematics, 21.09.2019 01:00












History, 21.09.2019 01:00


Social Studies, 21.09.2019 01:00


History, 21.09.2019 01:00

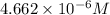 .
.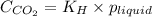
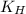 = Henry's constant =
= Henry's constant = 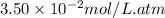
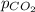 = partial pressure of carbonated drink
= partial pressure of carbonated drink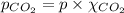
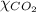 = mole fraction of
= mole fraction of