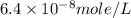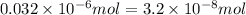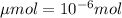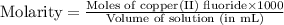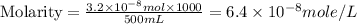Chemistry, 16.01.2020 20:31 starfox5454
Achemist prepares a solution of copper(ii) fluoride (cuf2) by measuring out 0.032 µmol of copper(ii) fluoride into a 500 ml volumetric flask and filling the flask to the mark with water. calculate the concentration in mol/l of the chemist's copper(ii) fluoride solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
You know the right answer?
Achemist prepares a solution of copper(ii) fluoride (cuf2) by measuring out 0.032 µmol of copper(ii)...
Questions

Social Studies, 09.07.2019 16:50

Mathematics, 09.07.2019 16:50

Chemistry, 09.07.2019 16:50

Mathematics, 09.07.2019 16:50

Mathematics, 09.07.2019 16:50


French, 09.07.2019 16:50

Chemistry, 09.07.2019 16:50