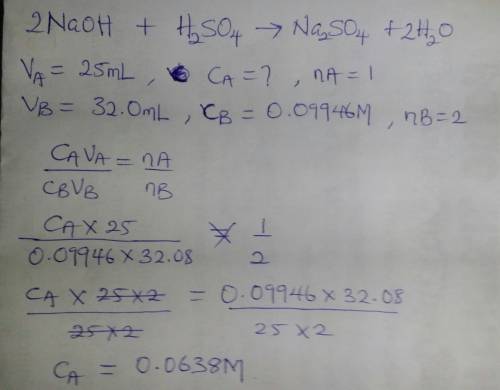Chemistry, 16.01.2020 04:31 risolatziyovudd
Asolution of sulfuric acid is titrated with a solution of sodium hydroxide to the phenolphthalein end point. the sample of sulfuric acid is 25.00 ml. the titration takes 32.08 ml of 0.09946 m sodium hydroxide.
a.) write out a balanced equation for the reaction.
b.) calculate the molarity of the sulfuric acid.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
Asolution of sulfuric acid is titrated with a solution of sodium hydroxide to the phenolphthalein en...
Questions





Mathematics, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01


Health, 16.10.2020 05:01


Mathematics, 16.10.2020 05:01


Chemistry, 16.10.2020 05:01


English, 16.10.2020 05:01

Spanish, 16.10.2020 05:01



Social Studies, 16.10.2020 05:01

History, 16.10.2020 05:01

Biology, 16.10.2020 05:01