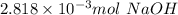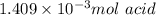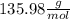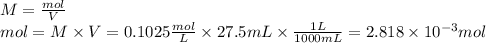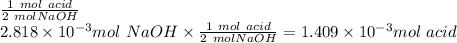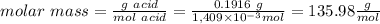Chemistry, 15.01.2020 19:31 eyeneedalife
1) a student dissolved 0.1916 g of an unknown diprotic acid in 100 ml of distilled water. the acid was then titrated with 0.1025m naoh solution. the second equivalence point showed the sharpest change in ph, and so it was used to determine the molar mass of the unknown acid. the volume of naoh needed to reach the equivalence point was 27.5 ml.
a. calculate the number of moles of naoh used in the titration to reach the second equivalence point.
b. calculate the number of moles of diprotic acid, based on the fact that we are examining the second equivalence point.
c. calculate the molar mass of the diprotic acid.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1
You know the right answer?
1) a student dissolved 0.1916 g of an unknown diprotic acid in 100 ml of distilled water. the acid w...
Questions

Mathematics, 24.02.2021 01:00

Chemistry, 24.02.2021 01:00

Mathematics, 24.02.2021 01:00


Mathematics, 24.02.2021 01:00

Law, 24.02.2021 01:00


Mathematics, 24.02.2021 01:00


Mathematics, 24.02.2021 01:00




History, 24.02.2021 01:00



English, 24.02.2021 01:00